Standard Curves To Determine The Unknown Solution

Introduction. A standard curve is a type of a graph which is used to establish the concentration of substance that is “Unknown”. To find out the concentration of an unknown solution, a spectrophotometer is used. Materials/methods. In order to perform the experiment, 9 tubes were used.
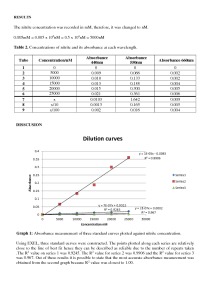
A standard curve is a type of a graph which is used to establish the concentration of substance that is “unknown”. When absorbance and concentration are in direct proportion, the line of best fit will be a straight line through the origin. An important substance in this research was nitrite. For each particular concentration of nitrite, the wavelength of light is varied such that three absorbance values are collected.
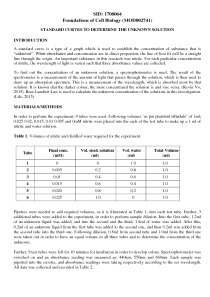
In order to perform the experiment, 9 tubes were used. Following volumes ‘as per practical schedule’ of 1ml; 0.025 0.02, 0.015, 0.01 0.005 and 0mM nitrite were placed into the each of the test tube to make up a 1 ml of nitrite and water solution.
Table 1: Volumes of nitrite and distilled water required for the experiment.
Pipettes were needed to add required volumes, as it is illustrated in Table 1, into each test tube. Further, 3 additional tubes were added to the experiment, in order to perform sample dilution. Into the first tube, 1.2ml of an unknown liquid was added, and into the second and the third, 1.
- Chemistry Laboratory works
- Microsoft Word 32 KB
- 2018 m.
- English
- 4 pages (1082 words)
- University
- Margarita